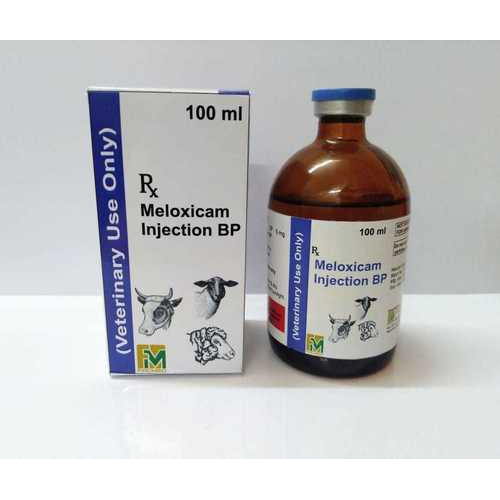
Meloxicam Injection Veterinary
Meloxicam Injection Veterinary Trade Information
- Minimum Order Quantity
- 1000 Packs
- Main Export Market(s)
- Asia
- Main Domestic Market
- All India
- Certifications
- WHO, GMP, ISO
About Meloxicam Injection Veterinary
Presentation:
One ml of the solution for injection contains 5 mg meloxicam as active ingredient and 150 mg anhydrous ethanol as preservative.
Uses
Metacam is a non-steroidal anti-inflammatory drug (NSAID) for use in dogs and cats.
Dogs:
Alleviation of inflammation and pain in both acute and chronic musculo-skeletal disorders. Reduction of post-operative pain and inflammation following orthopaedic and soft tissue surgery.
Cats:
Reduction of post-operative pain after ovariohysterectomy and minor soft tissue surgery.
Dosage and administration
Dogs
Musculo-skeletal disorders
Single subcutaneous injection at a dosage of 0.2 mg meloxicam/kg body weight (i.e. 0.4 ml/10 kg body weight).
Metacam 1.5 mg/ml Oral Suspension for Dogs or Metacam 1.0 mg and 2.5 mg Chewable Tablets for Dogs may be used for continuation of treatment at a dosage of 0.1 mg meloxicam/kg body weight, 24 hours after administration of the injection.
Reduction of post-operative pain (over a period of 24 hours)
Single intravenous or subcutaneous injection at a dosage of 0.2 mg meloxicam/kg body weight (i.e. 0.4 ml/10 kg body weight) before surgery, for example at the time of induction of anaesthesia.
Cats
Reduction of post-operative pain
Single subcutaneous injection at a dosage of 0.3 mg meloxicam/kg body weight (i.e. 0.06 ml/kg body weight) before surgery, for example at the time of induction of anaesthesia.
Particular care should be taken with regard to the accuracy of dosing.
Avoid introduction of contamination during use.
Contra-indications, warnings, etc
Do not use in pregnant or lactating animals.
Do not use in animals suffering from gastrointestinal disorders such as irritation and haemorrhage, impaired hepatic, cardiac or renal function and haemorrhagic disorders.
Do not use in case of hypersensitivity to the active substance or to any of the excipients.
Do not use in animals less than 6 weeks of age nor in cats of less than 2 kg.
Avoid use in any dehydrated, hypovolaemic or hypotensive animal, as there is a potential risk of renal toxicity.
During anaesthesia, monitoring and fluid therapy should be considered as standard practice. Any oral follow-up therapy using meloxicam or other Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) should not be administered in cats, as appropriate dosage regimens for such follow-up treatments have not been established.
Typical adverse reactions of NSAIDs such as loss of appetite, vomiting, diarrhoea, faecal occult blood, lethargy and renal failure have occasionally been reported. In very rare cases elevated liver enzymes have been reported.
In dogs, in very rare cases, haemorrhagic diarrhoea, haematemesis and gastrointestinal ulceration have been reported.
In dogs, these side effects occur generally within the first treatment week and are in most cases transient and disappear following termination of the treatment but in very rare cases may be serious or fatal.
In very rare cases anaphylactoid reactions may occur and should be treated symptomatically.
Pre-treatment with anti-inflammatory substances may result in additional or increased adverse effects and accordingly a treatment-free period with such veterinary medicinal products should be observed for at least 24 hours before commencement of treatment. The treatment-free period, however, should take into account the pharmacological properties of the products used previously.
Operator warnings
Accidental self-injection may give rise to pain. People with known hypersensitivity to NSAIDs should avoid contact with the veterinary medicinal product. In case of accidental self-injection, seek medical advice immediately and show the package leaflet or the label to the physician.
For animal treatment only.
Keep out of reach and sight of children.
Pharmaceutical precautions
Shelf life of broached vial: 28 days
Do not use after the expiry date stated on the carton and on the bottle.
Do not store above 25C.
Packaging Quantities
Colourless glass injection vials of 10ml or 20ml sealed with a rubber stopper and aluminium cap.
Storage Conditions:
Store it at room temperature.
Key Feature:
- WHO-GMP Certified
- Packaging can be customize
- Hygienically packed
- Balanced composition
- Stability
- Highly effective

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Pharmaceutical Medicines Category
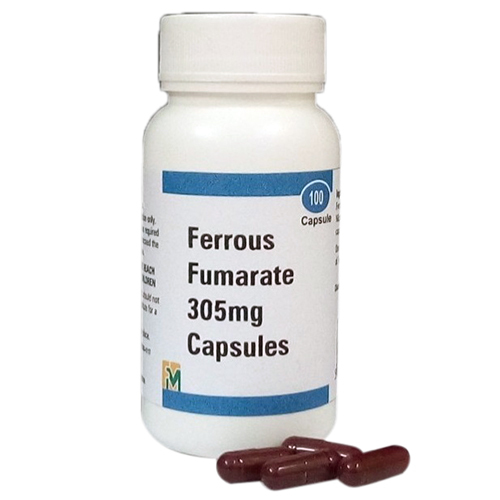
Ferrous Fumarate Capsules
Price 55 INR / Bottle
Minimum Order Quantity : 300 Bottles
Dosage Guidelines : As per physician
Drug Type : Generic Drugs
Physical Form : Capsules
Dosage : Ferrous fumarate is used to treat iron deficiency anemia (a lack of red blood cells caused by having too little iron in the body). Take ferrous fumarate on an empty stomach, at least 1 hour before or 2 hours after a meal.
Levonorgestrel Tablets
Price 12 INR / Pack
Minimum Order Quantity : 300 Boxes
Dosage Guidelines : As per physician
Drug Type : General Medicines
Physical Form : Tablets
Dosage : Levonorgestrel 0.75 mg tablet is an emergency contraceptive that can be used within 72 hours (3 days) of unprotected sex or if your usual contraceptive method has failed. It is about the following cases: No contraception was used during the sexual intercourse. The contraception measure was used incorrectly, for example as if a condom was penetrated, slipped away or used in a wrong way, if pessary or diaphragm changed position, burst, was broken or taken out ahead of time, in the case of a failed interruption during coitus interruptus (e.g. sperm ejaculated in or on external genitalia).
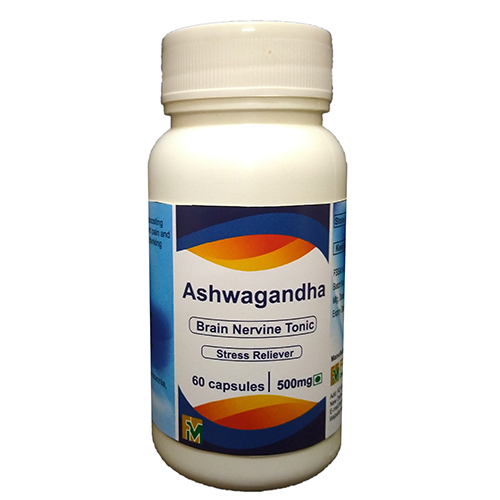
Ashwgandha Capsule
Price 300 INR / Bottle
Minimum Order Quantity : 3000 Bottles
Dosage Guidelines : As per doctor
Drug Type : General Medicines
Physical Form : Capsules
Dosage : As per doctor
 |
FACMED PHARMACEUTICALS PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |