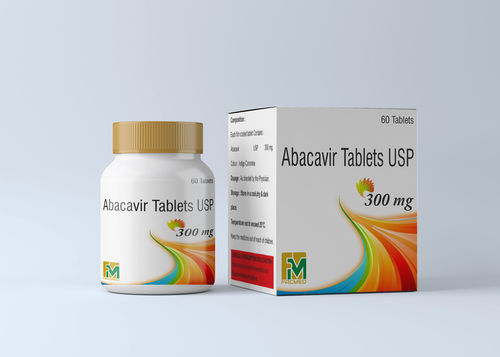
Abacavir Sulphate Tablets
Product Details:
- Dosage Form Tablets
- Origin of Medicine India
- Indication Abacavir tablets USP 300 mg, in combination with other antiretroviral agents, are indicated for the treatment of human immunodeficiency virus (HIV-1) infection. The recommended dosage of abacavir sulfate for adults is 600 mg daily, administered orally as either 300 mg twice daily or 600 mg once daily, in combination with other antiretroviral agents. The recommended dosage of abacavir sulfate in HIV-1-infected pediatric patients aged 3 months and older is 8 mg per kg orally twice daily (up to a maximum of 600 mg daily) in combination with other antiretroviral agents.
- Salt Composition Abacavir tablets USP 300 mg, in combination with other antiretroviral agents, are indicated for the treatment of human immunodeficiency virus (HIV-1) infection. The recommended dosage of abacavir sulfate for adults is 600 mg daily, administered orally as either 300 mg twice daily or 600 mg once daily, in combination with other antiretroviral agents. The recommended dosage of abacavir sulfate in HIV-1-infected pediatric patients aged 3 months and older is 8 mg per kg orally twice daily (up to a maximum of 600 mg daily) in combination with other antiretroviral agents.
- Pacakaging (Quantity Per Box) 30 tablets per box
- Drug Type Specific Drug
- Ingredients Abacavir tablets USP 300 mg, in combination with other antiretroviral agents, are indicated for the treatment of human immunodeficiency virus (HIV-1) infection. The recommended dosage of abacavir sulfate for adults is 600 mg daily, administered orally as either 300 mg twice daily or 600 mg once daily, in combination with other antiretroviral agents. The recommended dosage of abacavir sulfate in HIV-1-infected pediatric patients aged 3 months and older is 8 mg per kg orally twice daily (up to a maximum of 600 mg daily) in combination with other antiretroviral agents.
- Click to View more
Abacavir Sulphate Tablets Price And Quantity
- 500 Box
Abacavir Sulphate Tablets Product Specifications
- Anti-Viral
- Abacavir tablets USP 300 mg, in combination with other antiretroviral agents, are indicated for the treatment of human immunodeficiency virus (HIV-1) infection. The recommended dosage of abacavir sulfate for adults is 600 mg daily, administered orally as either 300 mg twice daily or 600 mg once daily, in combination with other antiretroviral agents. The recommended dosage of abacavir sulfate in HIV-1-infected pediatric patients aged 3 months and older is 8 mg per kg orally twice daily (up to a maximum of 600 mg daily) in combination with other antiretroviral agents.
- 300mg Boxes
- Abacavir tablets USP 300 mg, in combination with other antiretroviral agents, are indicated for the treatment of human immunodeficiency virus (HIV-1) infection. The recommended dosage of abacavir sulfate for adults is 600 mg daily, administered orally as either 300 mg twice daily or 600 mg once daily, in combination with other antiretroviral agents. The recommended dosage of abacavir sulfate in HIV-1-infected pediatric patients aged 3 months and older is 8 mg per kg orally twice daily (up to a maximum of 600 mg daily) in combination with other antiretroviral agents.
- Abacavir tablets USP 300 mg, in combination with other antiretroviral agents, are indicated for the treatment of human immunodeficiency virus (HIV-1) infection. The recommended dosage of abacavir sulfate for adults is 600 mg daily, administered orally as either 300 mg twice daily or 600 mg once daily, in combination with other antiretroviral agents. The recommended dosage of abacavir sulfate in HIV-1-infected pediatric patients aged 3 months and older is 8 mg per kg orally twice daily (up to a maximum of 600 mg daily) in combination with other antiretroviral agents.
- As per physician
- 30 tablets per box
- Adults Suitable For All
- Store it at room temperature
- Abacavir tablets USP 300 mg, in combination with other antiretroviral agents, are indicated for the treatment of human immunodeficiency virus (HIV-1) infection. The recommended dosage of abacavir sulfate for adults is 600 mg daily, administered orally as either 300 mg twice daily or 600 mg once daily, in combination with other antiretroviral agents. The recommended dosage of abacavir sulfate in HIV-1-infected pediatric patients aged 3 months and older is 8 mg per kg orally twice daily (up to a maximum of 600 mg daily) in combination with other antiretroviral agents.
- Abacavir tablets USP 300 mg, in combination with other antiretroviral agents, are indicated for the treatment of human immunodeficiency virus (HIV-1) infection. The recommended dosage of abacavir sulfate for adults is 600 mg daily, administered orally as either 300 mg twice daily or 600 mg once daily, in combination with other antiretroviral agents. The recommended dosage of abacavir sulfate in HIV-1-infected pediatric patients aged 3 months and older is 8 mg per kg orally twice daily (up to a maximum of 600 mg daily) in combination with other antiretroviral agents.
- Specific Drug
- Tablets
- Tablets
- India
Abacavir Sulphate Tablets Trade Information
- Cash in Advance (CID)
- 500000 Box Per Month
- 30 Days
- Sample costs shipping and taxes has to be paid by the buyer
- Abacavir tablets USP 300 mg tablet is packed in box and customized packaging is also available.
- Asia Australia Central America North America South America Eastern Europe Western Europe Middle East Africa
- All India
- WHO,GMP,ISO
Product Description
Abacavir Sulphate Tablets
Generic Name: Abacavir
Product Name: Abacavir 300 mg
Description :-
Abacavir tablets USP 300 mg, in combination with other antiretroviral agents, are indicated for the treatment of human immunodeficiency virus (HIV-1) infection. The recommended dosage of abacavir sulfate for adults is 600 mg daily, administered orally as either 300 mg twice daily or 600 mg once daily, in combination with other antiretroviral agents. The recommended dosage of abacavir sulfate in HIV-1-infected pediatric patients aged 3 months and older is 8 mg per kg orally twice daily (up to a maximum of 600 mg daily) in combination with other antiretroviral agents.
Abacavir tablet is also available as a scored tablet for HIV-1-infected pediatric patients weighing greater than or equal to 14 kg for whom a solid dosage form is appropriate. Before prescribing abacavir tablets, children should be assessed for the ability to swallow tablets.
Swallow the tablets whole; do not split, chew, or crush them. If you are taking the chewable tablets, you may chew or swallow them whole.
Pack Size : 30 tabs
Certification: WHO-GMP Approved

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
Other Products in 'HIV Medicine' category
 |
FACMED PHARMACEUTICALS PVT. LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |